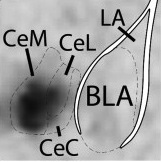
The dark smudge on the left side shows that the gene Tac2 is robustly activated after fear learning in the central amygdala. The amygdala is a part of the brain that controls the formation and consolidation of fear memories.
A drug that was previously tested in schizophrenia clinical trials is a potential option for targeting Tac2.
Scientists at Yerkes National Primate Research Center, Emory University have identified a drug that appears to make memories of fearsome events less durable in mice.
The finding may accelerate the development of treatments for preventing PTSD (post-traumatic stress disorder). The drug, called osanetant, targets a distinct group of brain cells in a region of the brain that controls the formation and consolidation of fear memories.
The results were published in the journal Neuron.
“Potentially, drugs that act on this group of cells could be used to block fear memory consolidation shortly after exposure to a trauma, which would aid in preventing PTSD,” says Kerry Ressler, MD, PhD, professor of psychiatry and behavioral sciences at Emory University School of Medicine and Yerkes National Primate Research Center. “PTSD is unique among psychiatric disorders in that we know when it starts – at the time of the trauma. Finding ways to prevent its development in the first place – in the emergency department or the battlefield - is an important and exciting avenue of research in this area.”
The first author of the paper is postdoctoral fellow Raül Andero Galí, PhD. Ressler and Andero were sifting through a list of many genes that are activated in the brains of mice after they learn to become afraid of a sound, because the sound is paired with a mild electric shock. The researchers were probing for changes in the central amygdala, a region of the brain known to regulate fear learning.
Out of thousands of genes they examined, their “top gene” was Tachykinin 2 or Tac2. The Tac2 gene was turned on more strongly during fear learning in mice that were previously exposed to a model of traumatic stress.
“The Tac2 gene is robustly activated after fear learning and belongs to a pathway that can be specifically blocked with a drug,” Ressler says. “It was interesting that Tac2 is highly expressed in one particular part of the amygdala, but with low or no expression in other brain areas related to the formation of fear memories. Also, we found that the cells that express Tac2 are distinct from those other investigators had previously identified as being involved in fear expression.”
Tac2 is part of a family of messengers in the nervous system known as tachykinins. Drugs that block a product encoded by Tac2’s relative, Tac1, are antiemetics, often prescribed when someone is receiving chemotherapy for cancer.
Osanetant, which blocks the action of Tac2, has been tested in previous clinical studies for schizophrenia and was safe but not effective in addressing that disorder. It has not been tested in humans for PTSD prevention.
“Osanetant is a safe and well-tolerated drug in humans and could be potentially used to prevent PTSD when given shortly after trauma, although more research is needed,” Andero says.
Under the influence of osanetant, mice could still learn to become afraid of a sound paired with a shock, but the mice did not freeze as much in response to the sound a day later, even if the drug was given an hour after training.
“Our goal is to specifically impair emotional memories related to a traumatic event instead of all memories associated with it. Thus, the trauma and its circumstances are remembered but the consolidation of fear memories is impaired, which could decrease the likelihood of developing fear-related disorders,” Andero says.
The research was supported by the National Institutes of Mental Health (1R21MH101492-01, 1R01MH096764), National Institute for Neurological Disorders and Stroke Core Facilities grant (P30NS055077), the Office of Research Infrastructure Programs (Primate centers: P51OD11132 – formerly NCRR P51RR000165) and the Burroughs Wellcome Fund. Ressler is a Howard Hughes Medical Investigator.
Reference:
R. Andero, B.G. Dias and K.J. Ressler. A role for Tac2, NkB and Nk3 receptor in normal and dysregulated fear memory consolidation Neuron - in press, corrected proof (2014).
